Synthetic Corneal Transplant Offers Hope for Sight Loss
Mr. Sheraz Daya, Centre for Sight’s medical director is to perform the country’s first artificial corneal transplant, using a breakthrough technique that could restore the sight of thousands of people. This technique hopes to initially treat up to a dozen patients in a UK trial, following approval from ethics committees.

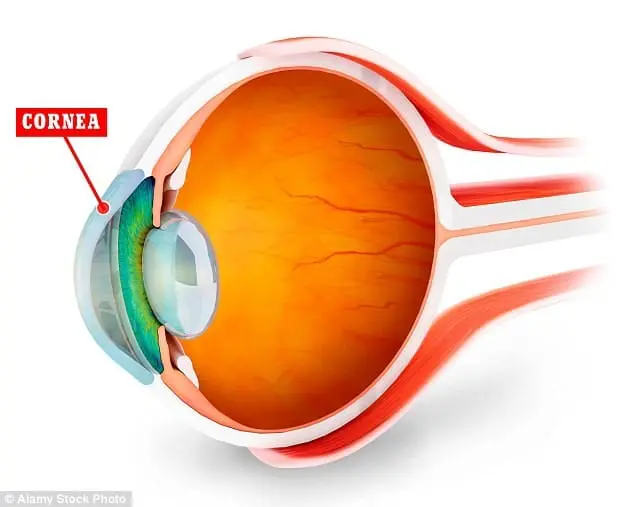
Previously, the only way to replace the transparent front part of the eye was to find a suitable donor. But donor corneas are in short supply and there is huge demand for them.
Now artificial collagen will be used to build a cornea that can be used for transplant. This gives hope to patients with badly impaired vision due to eye disease, injury or misshapen corneas. The first operations are expected early next year.
The artificial cornea will be made from synthetic collagen, grown in a laboratory using DNA techniques and will be similar to tissue found in the human body. Following the operation eye nerves and cells grow into the implanted artificial cornea, incorporating it into the eye.
Mr. Sheraz Daya who is also the medical director of LinCor Biomedical Sciences, said: “This technology could transform thousands of lives in this country and worldwide.” He also mentioned that the method would also avoid the risks from using human tissue of graft rejection and disease.
This is a welcome innovation and comes at a time when there is a corneal shortage in the UK. Donation of corneas decreased by 11% last year.
“There are 10 million people who have corneal blindness worldwide and many could benefit from this type of off the shelf cornea”
Mr. Daya

Elizabeth Keell, 36, a secondary school teacher from Kenilworth, Warwickshire, who has severely impaired vision, owing to degenerative corneal condition keratoconus, hopes to benefit. The mother of two said:
“My children rely on me so much and I need my sight. I welcome this new synthetic cornea.”
Author Information
Authored by Sheraz Daya MD FACP FACS FRCS(Ed) FRCOphth, Consultant Ophthalmic Surgeon & Medical Director, June 2019.
Net review due June 2026